Origins of specificity and promiscuity in metabolic networks, JBC
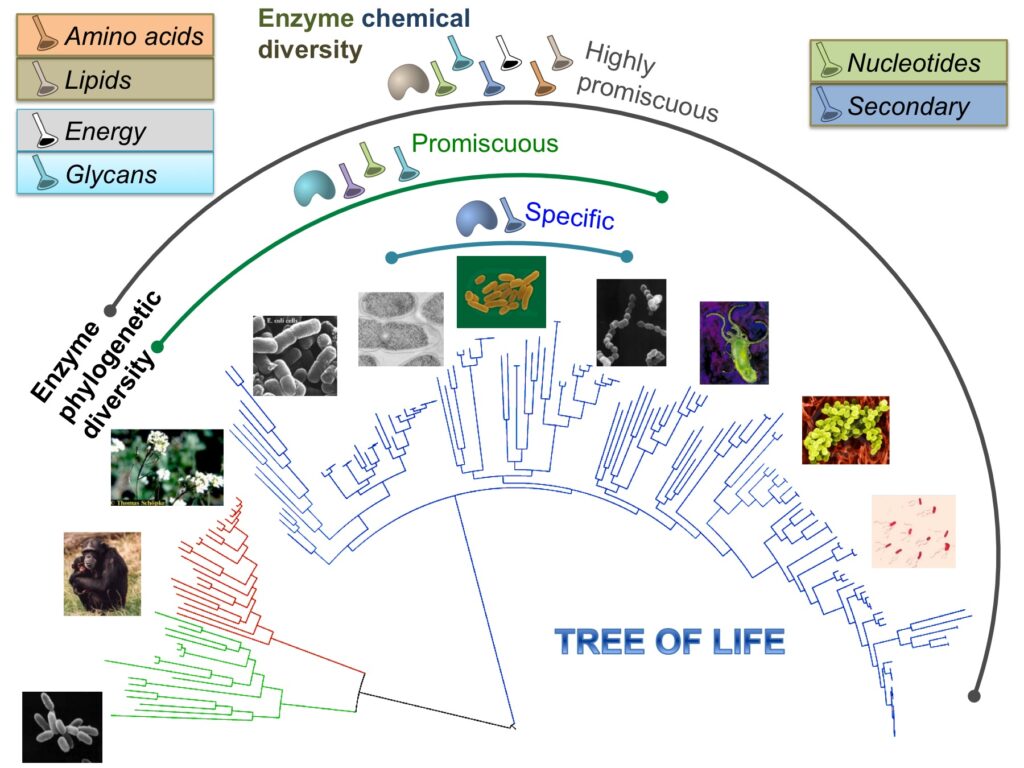 How enzymes have evolved to their present form is linked to the question of how pathways emerged and evolved into extant metabolic networks. To investigate this mechanism, we have explored the chemical diversity present in a largely unbiased dataset of catalytic reactions processed by modern enzymes across the tree of life. In order to get a quantitative estimate of enzyme chemical diversity, we measure enzyme multispecificity or promiscuity using the reaction molecular signatures.
How enzymes have evolved to their present form is linked to the question of how pathways emerged and evolved into extant metabolic networks. To investigate this mechanism, we have explored the chemical diversity present in a largely unbiased dataset of catalytic reactions processed by modern enzymes across the tree of life. In order to get a quantitative estimate of enzyme chemical diversity, we measure enzyme multispecificity or promiscuity using the reaction molecular signatures.
Our main finding is that reactions that are catalyzed by a highly specific enzyme are shared by poorly divergent species, suggesting a later emergence of this function during evolution. In contrast, reactions that are catalyzed by highly promiscuous enzymes are more likely to appear uniformly distributed across species in the tree of life. From a functional point of view, promiscuous enzymes are mainly involved in amino acid and lipid metabolisms, which might be associated with the earliest form of biochemical reactions. In this way, results presented in this paper might assist us into the identification of primeval promiscuous catalytic functions contributing to life’s minimal metabolism.
Carbonell P, Lecointre G, Faulon JL. Origins of specificity and promiscuity in metabolic networks, J Biol Chem, 2011. | doi: 10.1074/jbc.M111.274050



